Mechanisms of cardiac arrhythmias: from automaticity to re-entry
This chapter aims to present the most common arrhythmias in clinical practice. The initial discussion will focus on the mechanisms of cardiac arrhythmias. Although a detailed understanding of the mechanisms underlying cardiac arrhythmias is not necessary for all clinicians, it is wise to invest time in grasping the concepts as it will facilitate the understanding of subsequent articles and chapters. This chapter starts with a discussion on arrhythmogenesis (mechanisms of arrhythmias) and thereafter each arrhythmia is discussed separately.
It is appropriate to subdivide cardiac arrhythmias into the following groups:
- Bradyarrhythmias (bradycardia): arrhythmias that are typically due to dysfunctional automaticity in pacemaker cells or blocking of impulses somewhere along the conduction system.
- Supraventricular tachyarrhythmias (tachycardia): arrhythmias in which the impulses emanate from the atria.
- Ventricular tachyarrhythmias (tachycardia): arrhythmias in which the impulses emanate from the ventricles.
This classification is not flawless but it facilitates differential diagnostics and management of arrhythmias. Because the management of arrhythmias, particularly tachyarrhythmias, is often considered difficult, a special section is devoted to the diagnosis and management of arrhythmias. The recommendations presented throughout this section are in line with guidelines and recommendations issued by the European Society for Cardiology (ESC), the American Heart Association (AHA), and the American College of Cardiology (ACC).
Definition of heart rhythm
A rhythm is defined as three consecutive heartbeats displaying identical waveforms on the ECG. The similarity of the waveforms indicates that the origin of the impulse is the same. The sinoatrial (SA) node is the heart’s pacemaker under normal circumstances and the rhythm is referred to as sinus rhythm.
An arrhythmia is defined as an abnormal heart rhythm or heart rate, which is not physiologically justified. The latter criterion is important because rhythms that are physiologically justified should not be considered abnormal. For example, sinus bradycardia (a slow rhythm directed by the sinoatrial node) is a common finding in athletes and during sleep; in these scenarios, it should not be considered abnormal. On the other hand, sinus bradycardia developing during physical exercise is considered abnormal, because heart rate must increase during exercise.
Mechanisms of cardiac arrhythmias
The mechanisms underlying cardiac arrhythmias are being unraveled at an increasing pace. Arrhythmology is a very exciting area with intense research activity. This is partly due to the rise of cardiac imaging and invasive electrophysiological methods which enable detailed in vivo studies of arrhythmias. However, this chapter will not elaborate on research in arrhythmology; the discussions will be strictly clinical in order to provide the reader with robust knowledge of common arrhythmias. Readers who are interested in deeper studies are referred to Zipes et al (Clinical Arrhythmology, Elsevier, 2015).
Main causes of cardiac arrhythmias
Arrhythmias arise if the impulse formation is abnormal, if the impulse transmission is abnormal, or if both these are abnormal. These circumstances are now discussed in detail.
Abnormal impulse formation
Abnormal impulse formation can cause arrhythmias by the following two mechanisms:
- Increased or abnormal automaticity
- Triggered activity
Increased or abnormal automaticity
As discussed in Chapter 1 there are several structures in the heart that possess automaticity (i.e. the ability to depolarize spontaneously). These structures are as follows:
- The sinoatrial (SA) node: the sinoatrial node is the primary pacemaker of the heart. It directs the heart rhythm during normal circumstances and the rhythm is referred to as sinus rhythm.
- Parts of the atrial myocardium: There are clusters of atrial myocardial cells located around the crista terminalis, the entrance of the coronary sinus and the inferior vena cava, as well as cells around the mitral and tricuspid valves, which possess automaticity. These cells are not conduction cells per se; they are actually contractile cells that possess automaticity. Thus, automaticity is not exclusive to cells of the conduction system.
- Myocardium surrounding the atrioventricular (AV) node: It is a common misconception that the atrioventricular (AV) node possesses automaticity because there is no compelling evidence for that. There is, however, evidence that cell clusters surrounding the AV node possess automaticity. This automaticity will still – despite what has just been stated – be referred to as the automaticity of the AV node in order to facilitate understanding.
- The His-Purkinje network: The bundle of His and the entire Purkinje network possess automaticity.
These are the natural pacemakers of the heart because these structures possess automaticity, which is the intrinsic ability to depolarize spontaneously without previous stimulation. The intrinsic rate of spontaneous depolarization in these pacemaker structures follows:
- Sinoatrial node: 70 depolarizations per minute.
- Atrial myocardium: 60 depolarizations per minute.
- Cells around the atrioventricular node: 40 depolarizations per minute.
- His-Purkinje network: 20–40 depolarizations per minute.
The reason that the sinoatrial node is the primary pacemaker is simply that it has the fastest automaticity. Heart rhythm is directed by the fastest pacemaker because that pacemaker will depolarize before the competing pacemakers and reset their “clocks” before they discharge an action potential. The list also indicates that automaticity diminishes gradually with the distance from the sinoatrial node. This stepwise decline in automaticity is referred to as the pacemaker hierarchy of the heart.
The sinoatrial node may become dysfunctional and fail to depolarize. This could potentially result in cardiac arrest but it rarely does, because the absence of sinoatrial impulses will allow for one of the other pacemakers to take over the heart rhythm. This behavior is the reason why the other pacemakers are often referred to as latent pacemakers. Any rhythm that replaces the sinus rhythm is referred to as an escape rhythm. In case the sinoatrial node is dysfunctional, an escape rhythm will most likely come from atrial myocardium, because it has the second-highest rate of spontaneous depolarization. If the atrial myocardium also fails to generate action potentials, it is likely that an escape rhythm will come from cells around the atrioventricular node and so on. Note that the ventricular myocardium does not possess automaticity.
The automaticity in the sinoatrial node increases during physical exercise. The increased automaticity is a normal reaction since the cardiac output must increase during exercise. This is an example of a normal (physiological) increase in automaticity. However, in certain circumstances, the automaticity in the sinoatrial node and the other latent pacemakers can increase without physiological motivation. Some examples follow:
- Automaticity in the sinoatrial node can increase without physiological motivation and cause sinus tachycardia at rest. This is called inappropriate sinus tachycardia.
- The automaticity in latent pacemakers can increase, for example, during hypoxia, whereby they start discharging action potentials at a higher rate than the sinoatrial node and thus take over the heart rhythm.
- Purkinje cells located around the ischemic zone during acute myocardial ischemia/infarction can increase their automaticity and initiate ventricular tachycardia.
As mentioned above, ventricular myocardium does not possess automaticity, and neither does the vast majority of atrial myocardium. However, during pathological circumstances, even these cells may start discharging action potentials.
In other words, any cell may acquire abnormal automaticity and cause extrasystoles (extra beats) and arrhythmias. A wide range of conditions may cause abnormal automaticity; for example myocardial ischemia, hypokalemia, digoxin, hypoxia, lung disease, disturbances in the autonomic nervous system, etc. These conditions cause abnormal automaticity by changing the resting membrane potential of the cell, bringing it closer to the threshold for depolarization.
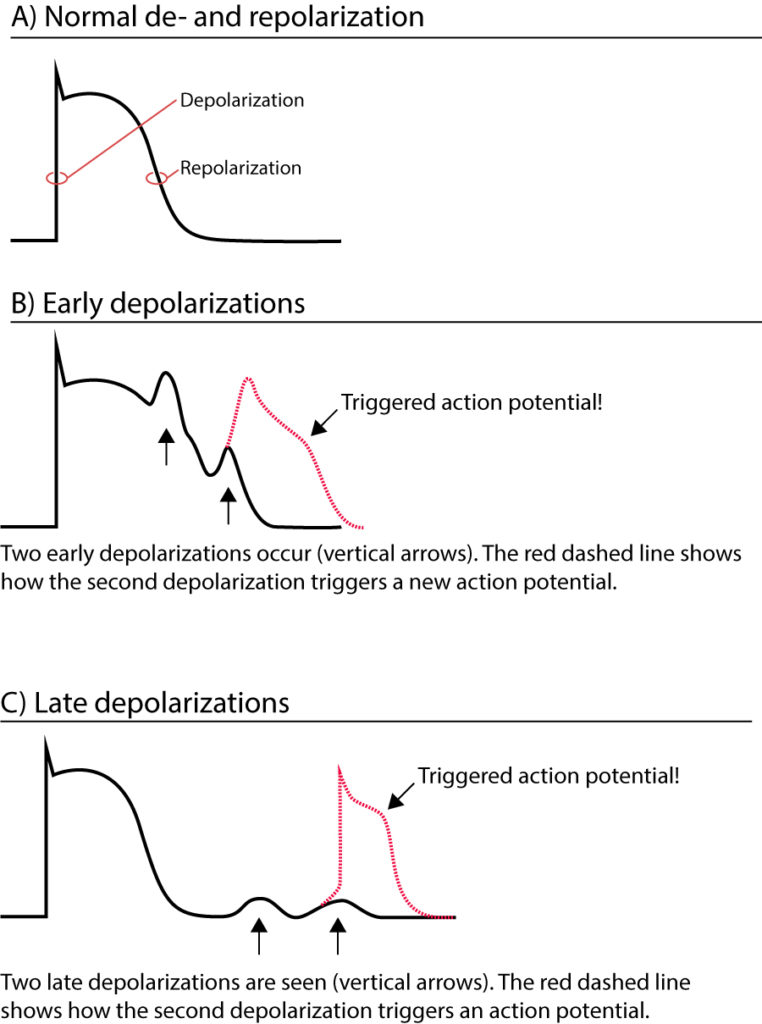
Triggered activity (after-depolarizations)
An action potential may induce an after-depolarization, which is a depolarization occurring either during or after the repolarization phase. An after-depolarization occurring during repolarization is referred to as an early depolarization, whereas after-depolarizations occurring after the repolarization are referred to as late depolarizations (Figure 1). Early and late depolarizations may be strong enough to reach the threshold for eliciting another depolarization. In other words, after-depolarizations may trigger action potentials. An action potential that is engendered by an after-depolarization is referred to as a triggered action potential. Such action potentials cause extrasystoles (extra heartbeats that fall in between the normal beats).
Early depolarizations are typically seen during bradycardia, hypokalemia, hypoxia, acidosis, hypocalcemia, and in drug side effects. Late depolarizations are seen in digoxin overdosing and during sympathetic stimulation.
Importantly, after-depolarizations may cause extrasystoles but they do not cause persistent arrhythmias. However, the extrasystoles might induce another arrhythmia mechanism (re-entry, see below) which may cause persistent arrhythmias.
Abnormal impulse conduction: re-entry (reentry)
Normal impulse transmission implies that the depolarizing wave spreads rapidly, uniformly, and unhindered through the myocardium. This requires that all cells ahead of the impulse wave are excitable and offer an equal capacity to transmit the impulse. Only under such circumstances can the depolarization (the impulse) spread through the myocardium like a wavefront in water. Should the impulse encounter cells that are not excitable or areas where the conductivity is heterogeneous, re-entry might occur.
It is fundamental to understand how re-entry occurs, as this mechanism is responsible for the majority of arrhythmias requiring treatment. The mechanism is somewhat intricate, but it can easily be understood with an illustration. Refer to Figure 2 and study it carefully. As seen in Figure 2, re-entry means that the depolarizing wavefront moves around itself in a circle. It is simply an electrical circle loop. This circular movement of the depolarizing wave is referred to as circus movement.
Re-entry occurs if the depolarizing impulse encounters a blocked area (“Central blocking” in Figure 2) that can only be passed on one side. The impulse manages to get around the central blocking on one side, circulates around it, and travels back. If the previously blocked area (blue area in Figure 2) has become excitable by the time the impulse arrives there, it will pass it. The depolarizing wavefront will then be able to continue this looping for as long as it encounters excitable tissue. This circus movement is typically very fast and it emits depolarizing impulses to the surrounding myocardium. Hence, the re-entry circuit generates impulses that activate the myocardium at a very high rate.
The prerequisites for re-entry have been noted in Figure 2. A brief explanation is repeated:
- There must exist a path of electrically connected myocardium. This path should form a circuit, around which the impulse can loop. Any cardiac cell that can carry out an action potential may be part of the circuit. The circuit may vary from a few millimeters to a decimeter in diameter.
- It is crucial that the cells included in the circuit have varying abilities to conduct the impulse. This variation is due to differences in refractoriness, conductivity and/or excitability, and it will lead to blocking of the arriving impulse.
- The circuit must surround a core of tissue that cannot be depolarized (central blocking). This core can consist of necrotic myocardium, scar tissue, or even valves (the valves have a fibrous ring).
Re-entry is subdivided into functional and anatomical. Knowledge of this distinction is not crucial for clinical practice.
Anatomical re-entry
The explanations outlined above actually apply to anatomical re-entry. In this type of re-entry, the central blocking consists of distinct anatomical structures. For example, atrial flutter (which is a re-entry tachyarrhythmia) arises when the impulse starts to circle around the tricuspid valve. In that scenario, the valve is the central blocking (valvular tissue cannot be depolarized) and the circuit is composed of the myocardial fibers surrounding the valve.
Anatomical re-entry is fixed, which means that the location of the re-entry and the speed by which it circulates is constant. It is also a stable re-entry; episodes of atrial flutter may persist for hours or even days. AVNRT (atrioventricular node re-entrant tachycardia), AVRT (atrioventricular re-entrant tachycardia), most cases of ventricular tachycardia (particularly those originating in the His-Purkinje network, as well as post-infarction cases) are also due to anatomical re-entry.
Functional re-entry
Functional re-entry is somewhat more difficult to grasp because the central blocking and the circuit around is more difficult to define anatomically. The central blocking and the circuit arise due to electrophysiological heterogeneity (variation) in the myocardium. Such heterogeneity includes varying refractoriness, conductivity and/or excitability. An impulse traveling through an area with such heterogeneity might encounter a functional block, circulate around it and during its first lap the wavefront will emit impulses both outwards and inwards (towards the core of the circuit). The core becomes bombarded with impulses and thus becomes refractory.
Functional re-entry circuits are small, unstable and may engender additional re-entry circuits (this is explained in the article on atrial fibrillation). Functional re-entry is fundamental for the development of atrial and ventricular fibrillation.
Clinical significance
Re-entry is the most common cause of supraventricular and ventricular arrhythmias that require treatment. Most cases of atrial flutter are due to re-entry and re-entry has a fundamental role in the development of atrial fibrillation. Re-entry can also occur in the sinoatrial node and atrioventricular node. Notably, ventricular tachycardia in persons with ischemic heart disease is caused by re-entry.
Termination of re-entry
The re-entry circuit will die out if the wavefront encounters tissue that cannot be excited (depolarized). The wavefront must continuously encounter excitable tissue in order to continue its movement. If it encounters non-excitable tissue it will be terminated. The purpose of delivering an electrical shock through the heart (for example during ventricular tachycardia) is to depolarize all excitable cells in the heart, including those involved in the re-entry, whereby the re-entry is terminated (the wavefront will encounter refractory cells).